Archives
Categories
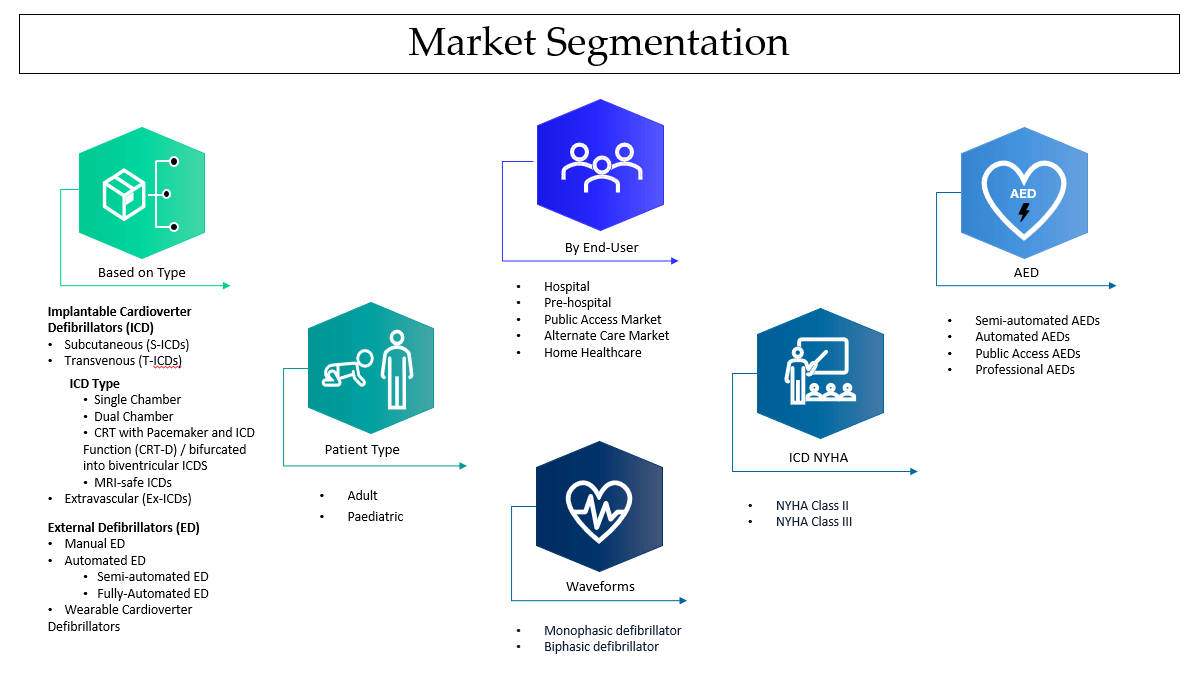
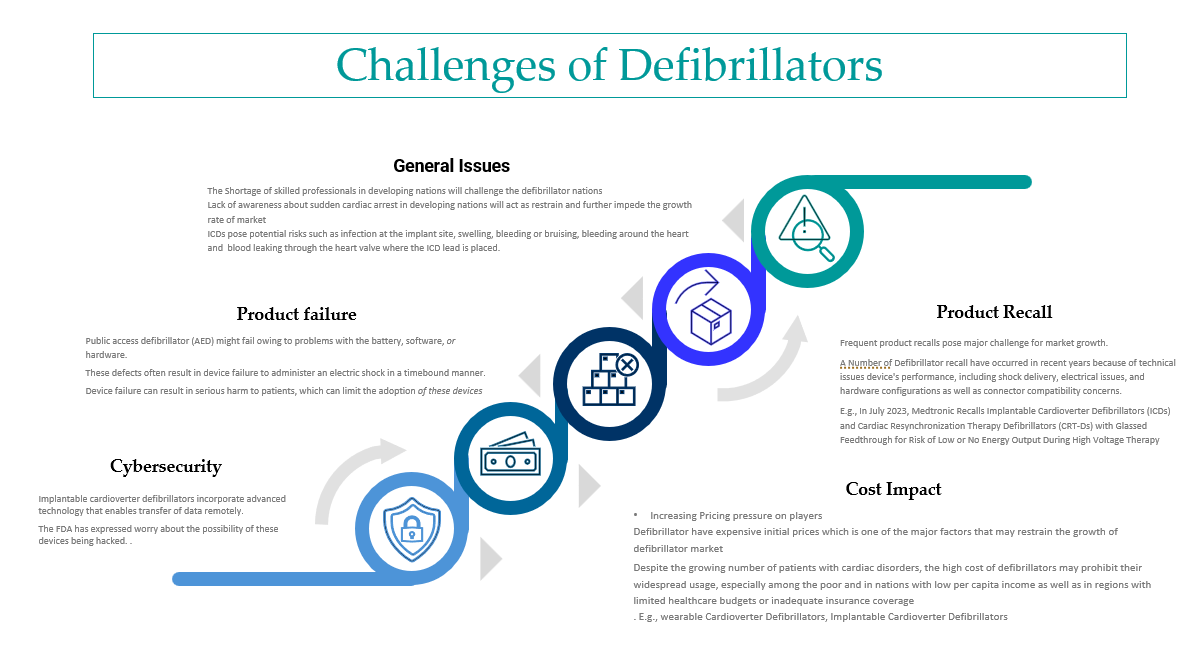
Defibrillator Market Research
This blog covers the size of the defibrillator market, market dynamics, drivers, trends, challenges, and the latest developments in the defibrillator space.
- Defibrillation is a process that involves administering electrocution to the heart, which depolarizes the cardiac muscles and recovers the heart’s standard electric impulse.
- Defibrillators is a medical device that is taken to be used for the purpose of delivering a shock or pulse electrically to the heart so that normal cycle is regained. Defibrillator delivers a therapeutic shock to a patient’s heart depending with the situation it is in like ventricular fibrillation, cardiac arrhythmia, and pulseless ventricular tachycardia.
Market Dynamics – Key Drivers
Increased number of cases/occurrences in various cardiac dysfunctions including Arrhythmia, coronary artery diseases, and cardiac failures.
» The Centers for Disease Control and Prevention (CDC) records show that in the year 2021, 695000 deaths in the United States were caused by CVD.
» According to the Scripps Health which is a non-profit health care system based in San Diego, California, estimates show that around 5 percent of the US population or 18 people suffer from an arrhythmia. Additionally, current studies indicate that a third of the American population of people above the age of forty may develop an abnormal heart rhythm.
Atrial fibrillation, ventricular tachycardia, supraventricular tachycardia (SVT), and ventricular fibrillation are anticipated to drive the market.
Growing Awareness about Cardiac Arrest, Resuscitation and Defibrillator
» Increasing public awareness about the importance of early defibrillation and effective cardiopulmonary resuscitation (CPR).
Recent Developments

In Jan 2024, Element Science got clearance for the product in Europe and a nod from the U. K. for a patch-based cardioverter defibrillator aimed at helping those in the danger zone of SCA to wear an easier patch instead of the old-style equipment.
FDA cleared their new EMBLEM MRI S-ICD system in Nov 2023 for Boston Scientific Corporation. This new subcutaneous implantable cardioverter defibrillator is MRI compatible, opening a new field for patients who require protection of a defibrillator and MRI scans at the same time.

The manufacturing and research facility for the Asia- Pacific region was recently launched by BIOTRONIK in November 2023.

October 2023: Medtronic has obtained FDA approval for a novel implantable cardioverter-defibrillator (Extravascular ICD), which the colossus refers to as the world’s only Extravascular ICD & for Epsila EV MRI SureScan defibrillation leads to address extremely fast heart rhythms that cause SCA.

In Oct 2023, the LIFEPAK CR3 defibrillator, which has a series of novel diagnostic tools and remote monitoring system to improve patients’ survival rate, is developed by Medtronic plc. Besides, following the CR2 model released in 2021, they have also introduced other models.